FORMA-05 Trial
Efficacy of fibrinogen concentrate in major abdominal surgery
Pseudomyxoma peritonei (PMP) is a rare malignancy with an estimated incidence rate of 1 to 3 people per million per year.1 It usually arises from a perforated primary appendix tumour with associated mucus production, resulting in the spread of mucus and tumour cells throughout the abdominal cavity. Although PMP does not usually metastasize systemically, it is eventually fatal in most cases unless definitively treated by surgery because the space required within the abdomen for nutritional function is replaced by progressive mucinous tumour and mucin accumulation.2
Cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) is the current established treatment for this peritoneal malignancy. It usually requires multiple peritonectomy procedures and visceral resections to remove malignant tissue from all involved intra-abdominal surfaces and organs, followed by intra-abdominal instillation of HIPEC for 1 hour at 42°C.2
CRS with HIPEC for extensive peritoneal malignancy, such as advanced PMP, may be associated with large-volume blood loss and substantial use of blood products such as plasma (e.g., 5-6 units) and red blood cells (RBC; e.g., 4 units). Mean blood loss has been estimated to be approximately 1500 mL (ranging up to approximately 5000 mL), with as many as three-quarters of patients requiring transfusions of allogeneic blood products. Effective coagulation management is, therefore, essential to help achieve successful outcomes.2
The main coagulopathy observed during this extensive surgery is the rapid fall in plasma fibrinogen concentration due to loss of whole blood, hemodilution and consumption through the extensive surgical excision. This acquired fibrinogen deficiency can only be corrected through administration of exogenous fibrinogen.
Two main sources of fibrinogen are cryoprecipitate and human fibrinogen concentrate (FC). Both product types have demonstrated ability to increase plasma fibrinogen levels in bleeding patients. However, cryoprecipitate has variable fibrinogen content, requires blood type matching, time to thaw, and carries risks of transfusion-related acute lung injury and pathogen transmission, and has been withdrawn in some European countries.3,4 In contrast, FC is a highly purified preparation that contains a defined concentration of fibrinogen, does not require blood type matching, and offers enhanced pathogen safety.5
Efficacy of fibrinogen concentrate in major abdominal surgery
A prospective, randomized, controlled study in cytoreductive surgery for pseudomyxoma peritonei
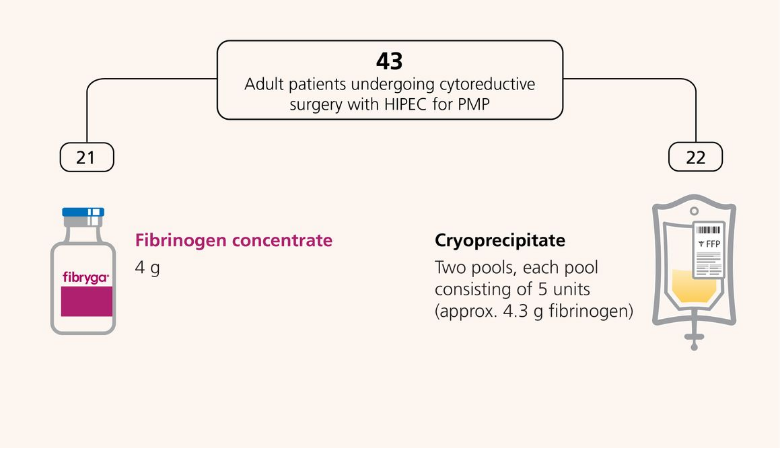
The FORMA 05, a prospective, randomized, controlled, open-label, phase 2 study, compared FC (fibryga®) with cryoprecipitate as sources of fibrinogen in patients undergoing cytoreductive surgery with HIPEC for PMP with predicted intraoperative blood loss ≥2 L.1
A total of 43 adult patients were randomised to receive FC (n=21) or Cryoprecipitate (n=22) in the per-protocol population (primary analysis population).
Primary objective
- To compare the haemostatic efficacy and safety of fibrinogen concentrate vs cryoprecipitate as a fibrinogen source for bleeding patients with acquired fibrinogen deficiency undergoing PMP CRS.
Treatment Intervention
Further FC or cryoprecipitate administration intraoperatively was based on the FIBTEM test of the ROTEM® analysis. A FIBTEM A20 of ≤12 mm triggered the administration of 4 g HFC or 2 pools of cryoprecipitate. Any administration of HFC or cryoprecipitate during the first 24 hours postoperatively was based on clinical judgment of the need for further haemostatic support and guided by FIBTEM analysis, with an A20 of ≤12 mm triggering administration of 2 g HFC or 1 pool of cryoprecipitate.