Top Questions on DOAC Reversal
Coming Soon!
-
Direct Oral Anticoagulants (DOACs) bind specifically to a factor in the coagulation pathway to inactivate them. They are generally oral medications that do not require monitoring for anticoagulation and have similar efficacy to warfarin.
- Other names: NOACs (“new” OACs), TSOACs (“target-specific” OACs)
-
Current DOACs are broadly divided into two categories:
- Direct thrombin inhibitors (inhibit factor 2): dabigatran
- Factor Xa inhibitors (inhibit activated factor 10): apixaban, edoxaban, rivaroxaban
-
General indications:
-
Prevention of stroke and embolism in atrial fibrillation patients
- Should NOT be used for prevention in patients with mechanical prosthetic heart valves
- Acute and extended treatment of venous thromboembolism (VTE)
- Prevention of VTE post-operatively (generally total knee or hip arthroplasty)
-
Prevention of stroke and embolism in atrial fibrillation patients
|
|
Direct Thrombin Inhibitors |
Factor Xa Inhibitors |
||
|
Dabigatran |
Apixaban |
Edoxaban |
Rivaroxaban |
|
|
Time to Peak Onset |
0.5-2 hours |
3-4 hours |
1-2 hours |
2-4 hours |
|
Half-life |
12-14 hours >24 hours if CrCl <30 mL/min |
8-15 hours |
10-14 hours |
5-9 hours 9-13 hours if elderly |
|
Drug Interactions |
P-glycoprotein |
CYP3A4 P-glycoprotein |
P-glycoprotein |
CYP3A4 P-glycoprotein |
|
Renal Excretion |
80% |
25% |
50% |
33% |
References:
- Brnjac E, Lin Y, Selby R. Chapter 2: Routine Coagulation Tests, Bloody Easy Coagulation Simplified (Second Edition). Ontario Regional Blood Coordinating Network. 2019.
- Shih AW, Crowther MA. Reversal of Direct Oral Anticoagulants: A Practical Approach. Hematology Am Soc Hematol Educ Program. 2016;612-619.
- Spahn DR, Beer JH, Borgeat A, et al. NOACs in Anesthesiology. Transfus Med Hemother. 2019;46:282-293.
- Eliquis® (Apixaban) Product Monograph, Pfizer Canada ULC. October 7, 2019.
- Lixiana® (Edoxaban) Product Monograph, Servier Canada Inc. July 26, 2017.
- Pradaxa® (Dabigatran) Product Monograph, Boehringer Ingelheim Canada Ltd. March 23, 2020.
- Xarelto® (Rivaroxaban) Product Monograph, Bayer Inc. September 20, 2019.
- Drug-specific serum levels to precisely test for DOAC levels are not commonly available, and thresholds of clinically significant cut-off values have not been validated with prospective trials
- Clinician understanding of assays available in local healthcare facilities is important to ensure appropriate result interpretation; consider consultation with laboratory pathologist
-
Direct thrombin inhibitors:
- Prothrombin Time (PT) and International Normalized Ratio (INR) – not useful
-
Activated Partial Thromboplastin Time (aPTT)
- When prolonged implies drug presence (highly specific)
- If normal, cannot exclude drug presence
-
Thrombin Time (TT) or Dilute Thrombin Time (dTT; Hemoclot®)
- When prolonged drug is present, even if a trivial amount
- If normal, definitively excludes drug presence (highly sensitive)
-
Factor Xa inhibitors:
-
PT/INR
- Rivaroxaban, edoxaban – When prolonged implies drug presence (specific); if normal, cannot exclude drug presence
- Apixaban – if normal, cannot exclude drug presence
- aPTT – not useful
-
Anti-Xa Level*
-
Level for factor Xa inhibition, specific for the anticoagulant used
-
- A positive test indicates that even a trivial amount of drug is present
- A negative test firmly rules out the presence of factor Xa inhibitor (sensitive test)
-
- *If drug-specific levels are not available, a negative test using unfractionated heparin or low-molecular weight heparin-calibrated assays rule out drug presence; a positive test indicates that drug is present
-
Level for factor Xa inhibition, specific for the anticoagulant used
-
PT/INR
Relative effect of DOAC presence in the plasma on commonly available coagulation assays, which yield qualitative results
|
|
Direct Thrombin Inhibitors |
Factor Xa Inhibitors |
||
|
Assay |
Dabigatran |
Apixaban |
Edoxaban |
Rivaroxaban |
|
PT/INR |
+/- |
+/- |
+/- |
++ |
|
aPTT |
++ |
- |
- |
- |
|
TT or dTT |
+++ |
- |
- |
- |
|
Anti-Xa Level |
- |
+++ |
+++ |
+++ |
+ implies a prolonged/abnormal value; - implies a normal value
References:
- Douxfils J, Ageno W, Samama CM, et al. Laboratory testing in patients treated with direct oral anticoagulants: a practical guide for clinicians. J Thromb Haemost. 2018;16:209-219.
- Samuelson BT, Cuker A, Siegal DM, et al. Laboratory assessment of the anticoagulant activity of direct oral anticoagulants: a systematic review. Chest. 2017;151:127-138.
Non-specific agents
-
In the setting of life-threatening bleeding due to factor Xa inhibitors, a dose of Prothrombin Complex Concentrates (PCC) 2000 IU was shown to have good hemostatic effectiveness in nearly 70% of patients (in observational studies)
- PCCs are thought to mitigate anti-Xa effect by increasing levels of non-activated clotting factors
- The ANNEXA-I trial comparing Andexanet alfa to usual care (85% of usual care arm received PCCs) in factor Xa inhibitor associated bleeding also demonstrated that PCCs have hemostatic efficacy (67% in Andexanet alfa arm and 53% in the usual care arm) (ANNEXA-I Randomized Controlled Trial)
- Adjunct therapies like tranexamic acid or topical hemostatic agents can be used to help manage DOAC-associated bleeding, but these are not DOAC reversal agents
Specific DOAC reversal agents
A "reversal agent" implies the presence of a specific antidote to counteract the effects of a drug
|
|
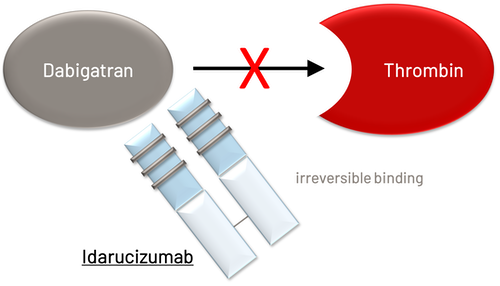
Direct Thrombin Inhibitor: Dabigatran |
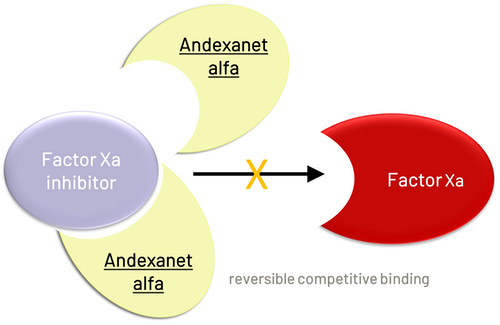
Factor Xa Inhibitors: Apixaban, Edoxaban, Rivaroxaban |
|
Reversal Agent |
Idarucizumab |
Andexanet alfa |
|
Health Canada Approved |
Yes |
Yes |
|
US FDA Approved |
Yes |
Yes |
|
EU EMA Approved |
Yes |
Yes |
|
Indication |
Adult patients treated with dabigatran requiring reversal of anticoagulation for emergency surgery/urgent procedures or life-threatening or uncontrolled bleeding |
Patients treated with apixaban or rivaroxaban requiring reversal of anticoagulation for life-threatening or uncontrolled bleeding (not yet indicated for edoxaban-treated patients) |
|
Mechanism |
Specific humanized monoclonal antibody fragment against dabigatran
|
Recombinant modified human factor Xa protein against factor Xa inhibitors
|
|
Dose |
5 g IV (2 x 2.5 g vials) |
Low Dose: 400 mg IV bolus at a target rate of 30 mg/min, followed by 4 mg/min IV infusion for up to 120 minutes (480 mg) High Dose: 800 mg IV bolus at a target rate of 30 mg/min, followed by 8 mg/min IV infusion for up to 120 minutes (960 mg) See the below chart to determine andexanet alfa dosing based on the specific factor Xa inhibitor dose (timing of factor Xa inhibitor last dose before andexanet alfa initiation) |
|
Time of Onset |
Immediate |
Immediate |
Andexanet alfa dose based on the specific factor Xa inhibitor dose (timing of factor Xa inhibitor last dose before andexanet alfa initiation):
|
Last Dose |
<8 Hours or Unknown |
≥8 Hours |
|
|
Apixaban |
≤10 mg |
Low dose |
Low Dose |
|
>10 mg or unknown |
High dose |
||
|
Rivaroxaban |
≤5 mg |
Low dose |
|
|
>5 mg or unknown |
High dose |
||
|
Edoxaban (off-label) |
≤30 mg |
Lose dose |
|
|
>30 mg |
High dose |
References:
- Andexxa® (Andexanet alfa) Package Insert. San Francisco, CA, USA; Portola Pharmaceuticals Inc. December 2018.
- Praxbind® (Idarucizumab) Product Monograph. Burlington, Ontario; Boehringer Ingelheim Canada Ltd. April 18, 2019.
- Carpenter E, Singh D, Dietrich E, et al. Andexanet alfa for reversal of factor Xa inhibitor-associated anticoagulation. Ther Adv Drug Saf. 2019;10:2042098619888133.
- Hoffman M, Goldstein JN, Levy JH. The impact of prothrombin complex concentrates when treating DOAC-associated bleeding: a review. Int J Emerg. 2018;11:55.
- Majeed A, Ågren A, Holmström M, et al. Management of rivaroxaban- or apixaban-associated major bleeding with prothrombin complex concentrates: a cohort study. Blood. 2017;130:1706-1712.
- Schulman S, Gross PL, Ritchie B, et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018;118:842-851.
General approach as follows:
-
Risk stratification should first occur, ideally with expert consultation
-
Assessment should include:
- Assessing hemodynamic stability
- Taking a bleeding history and determining the source of bleeding
- Medication history, including last dose and interactions
- Pre-existing anemia and renal failure
- Consider life-threatening bleeds, including: hemodynamic instability, transfusion of ≥3 units of red blood cells in 1 hour, end organ damage from anemia
-
Assessment should include:
- Stop the drug
- Consider activated charcoal if last dose within 2-3 hours for highest risk, most severe bleeds
-
Consider testing to rule in/out the presence of therapeutic DOAC levels ( See question #2)
- Do NOT wait until testing comes back before acting
-
Supportive care
- Includes wide-bore IV access and transfer to a monitored setting
-
Blood transfusion should occur similarly to non-DOAC bleeding
- Consider an Hb target of 70 g/L in GI bleeding
- Plasma is often not initially needed in liver disease/upper GI bleeding
- Plasma is not indicated for DOAC “reversal”
- Platelets should generally be transfused if below 50 x 109/L
- Check fibrinogen levels, if possible
-
Local hemostasis
- Mechanical compression and local hemostatic measures
- Surgical and interventional radiology procedures that definitively stop the bleeding, even with the use of coagulation products and reversal agents, are the cornerstones of therapy
-
Tranexamic acid
- Should be strongly considered in DOAC-associated bleeding, though it has not been studied specifically in this population
-
Specific DOAC reversal agents whenever possible ( See question #3)
- Dabigatran: idarucizumab
- Factor-Xa inhibitors (apixaban, edoxaban, rivaroxaban): andexanet alfa (not commonly available in Canada)
-
Non-specific agents to treat DOAC-associated bleeding – if specific DOAC reversal agents not available
- Dabigatran: hemodialysis and/or activated prothrombin complex concentrates (aPCCs) can be considered if idarucizumab not available, but rebound often occurs quickly after hemodialysis
-
Factor-Xa inhibitors (apixaban, edoxaban, rivaroxaban): PCCs 2000 IU (or 25-50 IU/kg to max 3000 IU) is likely the best alternative therapy without a specific reversal agent
- Precise dose is unknown due to lack of dose-ranging studies
-
Other non-specific agents/considerations
- Use of recombinant factor VIIa is not recommended, due to evidence of harm
- No evidence that frozen plasma transfusion has any effect in DOAC bleeding management
Surgical and interventional radiology procedures that definitively stop the bleeding, even with the use of coagulation products and reversal agents, are the cornerstones of therapy.
References:
- Cuker A, Burnett A, Triller D, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol. 2019;94:697-709.
- Piran S, Khatib R, Schulman S, et al. Management of direct factor Xa inhibitor-related major bleeding with prothrombin complex concentrate: a meta-analysis. Blood Adv. 2019;3:158-167.
- Schulman S. Bleeding Complications and Management on anticoagulant therapy. Semin Thromb Hemost. 2017;43:886-892.
- Shih AW, Crowther MA. Reversal of Direct Oral Anticoagulants: A Practical Approach. Hematology Am Soc Hematol Educ Program. 2016;612-619.
For non-life-threatening bleeds, follow the left side of the figure.
The determination of life-threatening versus non-life-threatening bleeding is a clinical decision based on patient stability and circumstances.
Examples of minor bleeding include most cases of epistaxis, ecchymosis, and heavy menstrual bleeding.
Most of these bleeds can be managed through temporary drug discontinuation and local hemostatic measures.
-
Drug discontinuation should weigh the balance between reducing bleeding and the risk of thromboembolism, ideally done in concert with expert consultation
- Dose reduction may also be considered with expert consultation
- Tranexamic acid should be considered, notably for recurrent bleeding
- Local interventions to stop bleeding should also be considered, depending on the risk of the procedure
If bleeding continues and there is clinical deterioration, ( See question #4)
References:
- Piran S, Khatib R, Schulman S, et al. Management of direct factor Xa inhibitor-related major bleeding with prothrombin complex concentrate: a meta-analysis. Blood Adv. 2019;3:158-167.
- Schulman S. Bleeding Complications and Management on anticoagulant therapy. Semin Thromb Hemost. 2017;43:886-892.
- Shih AW, Crowther MA. Reversal of Direct Oral Anticoagulants: A Practical Approach. Hematology Am Soc Hematol Educ Program. 2016;612-619.

Reversal of the patient's DOAC may be needed for those who require an emergency surgery (<8-12 h, e.g., intracranial bleed, ruptured viscus, cardiac tamponade), urgent surgery (<24 h, e.g., hip fracture repair, acute cholecystitis), or invasive procedure that cannot be delayed. Consider data of reversal and/or prophylactic treatment is limited for Xa inhibitors.
Obtain the following information:
- Which DOAC?
- What dose?
- When was the last dose?
- Most recent creatinine?
-
Is it a major surgery – high risk of bleeding or severe consequences of bleeding? If Yes
-
How long can the surgery safely be delayed? – Necessary within 24 h? If Yes
- Assay to measure DOAC levels (if available?) ( See question #2)
- Estimate creatinine clearance (eCrCl) using Cockroft-Gault formula
-
How long can the surgery safely be delayed? – Necessary within 24 h? If Yes
Dabigatran
- If thrombin time is normal or dilute thrombin time (Hemoclot®) <50 ng/mL – OK to operate
- If dilute thrombin time unavailable – use timing of last dose and eCrCl to determine presence of drug – idarucizumab can effectively reverse the anticoagulant effect of dabigatran if needed ( See question #3):
|
Last Dose |
eCrCl >50mL/min |
eCrCl 30-50mL/min |
eCrCl <30mL/min |
|
<48 h ago |
Idarucizumab 5 g |
Idarucizumab 5 g |
Idarucizumab 5 g |
|
48-96 h ago |
OK to operate |
Idarucizumab 5 g |
Idarucizumab 5 g |
|
96-144 h ago |
OK to operate |
OK to operate |
Idarucizumab 5 g |
Apixaban, edoxaban, or rivaroxaban
- If anti-Xa level calibrated for the specific DOAC is <50 ng/mL – OK to operate
- If anti-Xa activity assay unavailable, use timing of last dose and eCrCl to determine presence of drug. PCCs 2000 IU is likely the best therapy without a specific reversal agent (Andexanet alfa not commonly available in Canada) ( See question #3):
|
Last Dose |
eCrCl >50mL/min |
eCrCl 30-50mL/min |
eCrCl <30mL/min |
|
<48 h ago |
PCC 2000 units* |
PCC 2000 units* |
PCC 2000 units* |
|
48-96 h ago |
OK to operate |
OK to operate |
PCC 2000 units* |
|
96-144 h ago |
OK to operate |
OK to operate |
OK to operate |
*No clinical evidence
Or use the electronic tool ( Perioperative Anticoagulant Management Algorithm).
For guidance on the perioperative management of patients taking a DOAC before an elective surgery/procedure, please visit the following Thrombosis Canada guide.
References:
- Cuker A, Burnett A, Triller D, et al. Reversal of direct oral anticoagulants: Guidance from the Anticoagulation Forum. Am J Hematol. 2019;94:697-709.
- Douketis JD, Spyropoulos AC, Duncan J, et al. Perioperative management of patients with atrial fibrillation receiving a direct oral anticoagulant. JAMA Intern Med. 2019;179:1469-1478.
- Macle L, Cairns JA, Andrade JG, et al. The 2014 Atrial Fibrillation Guidelines Companion: A Practical Approach to the Use of the Canadian Cardiovascular Society Guidelines. Can J Cardiol. 2015;31:1207-1218.
- Shaw JR, Woodfine JD, Douketis J, et al. Perioperative interruption of direct oral anticoagulants in patients with atrial fibrillation: A systematic review and meta-analysis. Res Pract Thromb Haemost. 2018;2:282-290.
- DOAC: Management of Bleeding, Thrombosis Canada Clinical Guide (Clinical Guides - DOACs: Management of Bleeding | Thrombosis Canada)
For information on DOAC reversal agents, See question #3
Direct thrombin inhibitor (dabigatran) associated bleeding after idarucizumab administration:
-
Administration of an additional dose of idarucizumab 5 g may be considered if:
- Clinically significant bleeding together with an increase in thrombin time occurs 12-24 hours after administration of the standard dose of idarucizumab 5 g
Factor Xa inhibitor (apixaban, edoxaban, rivaroxaban) associated bleeding after PCC administration:
-
Administration of an additional dose of PCC may be considered if:
- Visible evidence of bleeding 4 hours or more after the initial PCC dose administration
- Patient clinical condition has deteriorated with clinical evidence of poor hemostasis (e.g., >20% decrease in both Hb and Hct, or evidence of significant hematoma expansion on imaging within 24 hours from the initial PCC dose administration)
Factor Xa inhibitor (apixaban, edoxaban, rivaroxaban) associated bleeding after andexanet alfa administration:
- The safety and efficacy of more than one dose has not been clinically evaluated and cannot be recommended by the manufacturer
- PCC use may be considered, but consideration must be given to balancing thrombosis risk in the patient
References:
- Andexxa® (Andexanet alfa) Package Insert. San Francisco, CA, USA; Portola Pharmaceuticals Inc. December 2018.
- Praxbind® (Idarucizumab) Product Monograph. Burlington, Ontario; Boehringer Ingelheim Canada Ltd. April 18, 2019.
- Schulman S, Gross PL, Ritchie B, et al. Prothrombin complex concentrate for major bleeding on factor Xa inhibitors: a prospective cohort study. Thromb Haemost. 2018;118:842-851.
Consultation with a hematologist or thrombosis expert is strongly recommended before re-starting DOAC in someone who has experienced a life-threatening bleed.
References:
- Majeed A, Kim Y, Holmström M, Roberts RS, Schulman S. Optimal timing of resumption of warfarin after intracranial hemorrhage. Stroke. 2010;41:2860-2866.
- Majeed A, Wallvik N, Eriksson J, Hoijer J, Bottai M, Holmström M, Schulman S. Optimal timing of vitamin K antagonist resumption after upper gastrointestinal bleeding. A risk modelling analysis. Thromb Haemost. 2017;117:491-499.
- Pennlert J, Overholser R, Asplund K, et al. Optimal timing of anticoagulant treatment after intracerebral hemorrhage in patients with atrial fibrillation. Stroke. 2017;48:314-320.
- Schulman S. Bleeding Complications and Management on anticoagulant therapy. Semin Thromb Hemost. 2017;43:886-892.
A practical, evidence-based guide for front-line physicians on how to treat acquired bleeding
Important Disclaimer
This feature is intended for educational use only by healthcare professionals. Please verify the suggestions before considering use in patient care settings. For further information please consult our Terms of Use document. If you agree with these terms, and with the educational nature of this website then please acknowledge below.